Hongwei Wang’s group made breakthroughs in studying the structural mechanisms of important drug-target G protein-coupled receptor (GPCR) complexes by cryo-EM in recent collaborations with the Brian Kobilka group and Amgern company.
In humans, there are more than 800 GPCRs which make up the largest membrane protein superfamily. GPCRs play important roles for cells in sensing the extracellular signals, such as odors, photon, hormones, cytokines and transmitters. Activated GPCRs participate in different biological processes through coupling to variant G proteins and subsequently variant signaling pathways, making GPCRs potential targets for drug development. Currently, more than 40% of clinically used modern drugs function on GPCRs, and many novel drugs targeting GPCRs are in different stages of clinical test. Despite the highly conserved sequence and especially three-dimensional structures among GPCRs, the interaction between each ligand and its specific GPCR is quite different. In drug development, it is critical to enhance the binding specificity of a drug molecule on its GPCR target to reduce the side effects. Therefore, a high resolution structure of ligand-bound GPCR is important to provide the precide information in addressing the interaction between the drug molecule and its GPCR target. Indeed, structure-based novel drug screening and optimization has now become a powerful tool in drug discovery with high efficiency. Hongwei Wang’s group has been devoted in development of novel cryo-EM techniques and the application of cryo-EM to solve high-resolution structures of macromolecules, among which are important drug-target GPCR complexes.
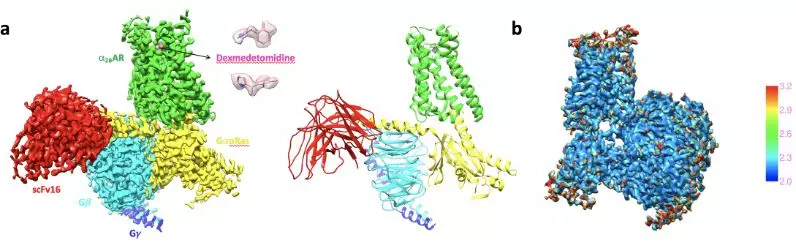
In collaboration with Prof. Brian Kobilka’s group from Stanford University, Prof. Wang’s group recently determined the high resolution cryo-EM structures of α2B adrenergic receptor complexed with Gi or Go protein at resolutions of 3.9 angstrom and 2.8 angstrom, respectively. The structures show clear solution of the ligand, dexmedetomidine, which is responsible to activate the α2B adrenergic receptor to bind with Gi or Go protein and subsequently induce the downstream signaling transduction. Dexmedetomidine interacts with α2B adrenergic receptor with high binding affinity and is widely used in hospitals as an analgesic and pain killer. However, Dexmedetomidine as a drug also induces many side effects because of its non-specific off-target property. In comparison with the reported cryo-EM structure of various GPCR complexes, the current structures solved by Wang and Kobilka groups present the highest resolution and the best-quality map of the density of dexmedetomidine captured in the α2B adrenergic receptor despite that the dexmedetomidine was one of the smallest ligands among all the GPCR complexes solved by cryo-EM so far. The accurate structural information of the interaction between dexmedetomidine and the receptor allows Peter Gmeiner’s group from Germany to perform a molecular dynamic simulation to further explain the activation mechanism of α2B adrenergic receptor. The results provide significant insights to further optimizing small molecules to target α2B adrenergic receptor with enhanced binding affinity and specificity. This work was recently published in Nature Chemical Biology. Dr. Daopeng Yuan from the School of medicine, Dr. Zhongmin Liu from the School of Life Sciences at Tsinghua University and Dr. Jonas Kaindl from Friedrich Alexander University are the co-first authors. Prof. Brian Kobilka from Stanford University, Prof. Hongwei Wang from Tsinghua university and Prof. Peter Gmeiner from Friedrich Alexander University are the co-corresponding authors.
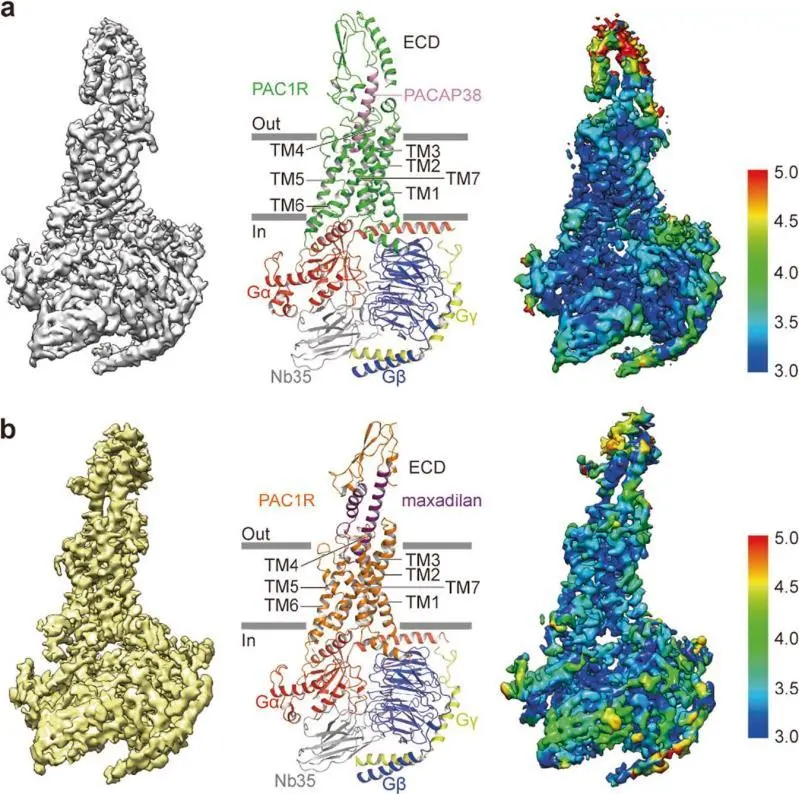
Prof. Wang’s group also solved the high resolution cryo-EM structures of PAC1 receptor complexes in collaboration with a research group at Amgen, one of the major pharmaceutical companies. PAC1R belongs to the serotine GPCR subfamily, widely expressed in the central nervous system and nerve endings. The dis-regulation of PAC1R could cause the activation and sensitization of trigeminovascular system and subsequently induce Migraine Headache, which makes PAC1R an important target for drug development. As agonists of PAC1R, PACAP and maxadilan are two polypeptides with distinguished amino acid sequences. Hongwei Wang group and their collaborators determined the high resolution cryo-EM structures of PAC1R complexed with PACAP38 and maxadilan, respectively. Based on the structural information of PAC1R complexes , two modes for activating PAC1R were analyzed, which has great significances in understanding the variant activating mechanisms of PAC1R by PACAP38 and maxadilan. The variant activating mechanisms provide a novel clue for designing drugs specifically targeting PAC1R. This work was recently published on Cell Research. Dr. Jia Wang from School of Life Sciences, Tsinghua University and Dr. Xianqiang Song from the Amgen company are the co-first authors. Prof. Wang from Tsinghua university and Dr. Yingli Ma from Amgen company are the co-corresponding authors.
Both works on GPCR structures using cryo-EM showcased the capability of high-resolution cryo-EM in the modern drug development.